Engineering Immune Cells For Therapeutic Benefit
Development of tractable methods for CRISPR-based reprogramming of human immune cells opens the door to application of reprogrammed cellular therapies to cancer, HIV, primary immune deficiencies, and autoimmune diseases.
.png)

Our lab previously discovered, with Jennifer Doudna, a Cas9 ribonucleoprotein (RNP) technology for robust genome editing in primary human T cells. We have since developed a CRISPR genome-targeting system independent of viral vectors, allowing rapid and efficient insertion of large DNA sequences at specific loci in primary human T cells. This strategy allows for correction of pathogenic mutations as well as replacement of loci such as the TCR to redirect human T cells to custom antigens. This methodology is being optimized and expanded to additional cell types, such as B cells, NK cells, and HSPCs.

Combining technology development with functional genomic discovery, the lab has developed methods for genome-wide CRISPR screens in human T cells (SLICE) as well as a complementary strategy to barcode and track targeted integrations of large DNA templates, enabling pooled knock-in screens. Broadly, these technologies are allowing us to probe DNA sequences that control cells of the human immune system, understand disease genetics, and enhance cellular immunotherapies. We are using these platforms to design next-generation chimeric antigen receptor (CAR) and TCR-engineered T cells to treat solid tumors refractory to current therapies, autoimmune diseases and infectious diseases.





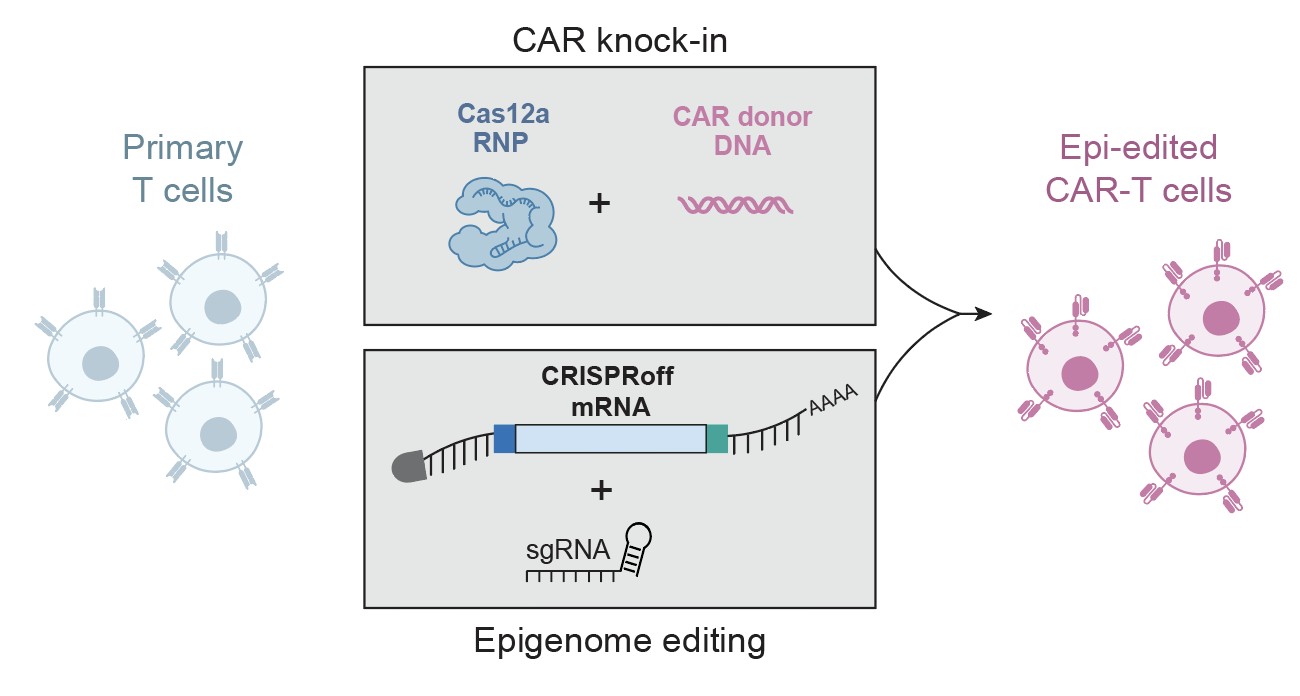



.jpg)


.jpg)
.jpg)
.jpg)
.webp)
.webp)
.jpg)
.png)
.jpg)


.svg)